December 12, 2013
Status Report for Quarter 3 - 2013 HCPCS Code A4253 Service-Specific Prepayment Review
The Medical Review Department of CGS, the Jurisdiction C DME MAC, began a service-specific prepayment edit for HCPCS code A4253 (Blood Glucose Test Strips) in 2009. This edit is the result of data demonstrating a high claims payment error rate for this product category. A summary report for claims reviewed between July 1, 2013 and September 30, 2013 follows:
| Current Quarter | Previous Quarter | |
|---|---|---|
| Denial Rate | 97% | 98% |
| Allowed Dollars Error Rate | 95% | 95% |
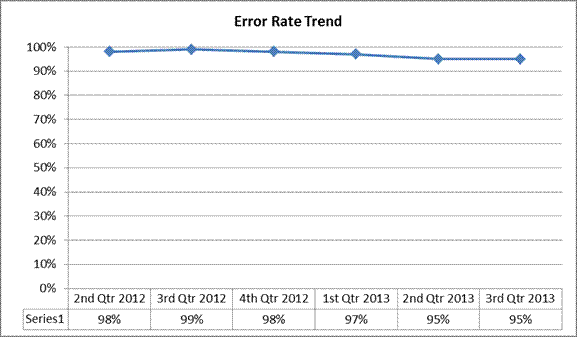
Non-response Rate to Additional Documentation Requests – 11%
An analysis of the claim denials from this review shows that the top 10 reasons a determination was made not to pay the claim were:
- Medical records were not provided.
- The refill request was missing the quantity of each item that the beneficiary still had on hand.
- Claim history showed that multiple suppliers were billing the same items for overlapping dates of service and another supplier had already been paid for the maximum quantity of medically necessary supplies for the time span under review.
- The detailed written order was missing one or more of the following elements: 1) Beneficiary's name, 2) Specific list of all items to be dispensed, 3) Specific frequency of testing, 4) Start date (if different from signature date).
- The documentation lacked a current (within six months prior to the date of service being reviewed) test log or physician record (narrative statement in the progress notes, etc.) documenting the frequency at which the beneficiary was actually testing.
- The medical records did not provide a beneficiary-specific explanation as to why additional testing materials were medically necessary.
- The documentation received did not include a copy of a valid refill request.
- Proof of delivery was not provided or was incomplete.
- The date of service entered on the claim did not match the shipping date.
- The refill request did not provide a description of the item(s) to be dispensed.
Case Study
R.E. – 73 year old male
DOS – 09/08/2013
Documentation in the file includes the following order:
| Patient Name & Address R.E. PO Box XXX Anytown, USA |
Date: June 1, 2013 |
Electronically signed by John Doe, M.D. on 06/01/2013 |
|
Claim history shows that the supplier previously billed for blood glucose testing supplies for DOS 06/02/13, 07/05/13 and 08/07/13.
Medical Review Decision – Deny the claim. A detailed written order for blood glucose monitor supplies must include the following information:
- Beneficiary's name
- Physician's name
- Date of the order and the start date (if different from the start date)
- Detailed description of the item(s) to be dispensed
- Frequency of use/testing frequency
- Quantity to be dispensed
- Number of refills
The frequency of use information must contain instructions for use and specific amounts to be dispensed. The above order is missing the quantity to be dispensed and the number of refills (or length of need).
The Medical Review Department of CGS began a service-specific Documentation Compliance Review (DCR) of HCPCS code A4253 (Blood Glucose Test Strips) claims on October 6, 2011. DCRs are nonclinical, technical reviews that evaluate the presence or absence of particular pieces of documentation. This type of review is conducted when data analysis indicates there is a pattern of insufficient documentation in a product category.
A summary report for claims reviewed between July 1, 2013 and September 30, 2013 follows:
| Current Quarter | Previous Quarter | |
|---|---|---|
| Denial Rate | 65% | 80% |
| Allowed Dollars Error Rate | 71% | 77% |
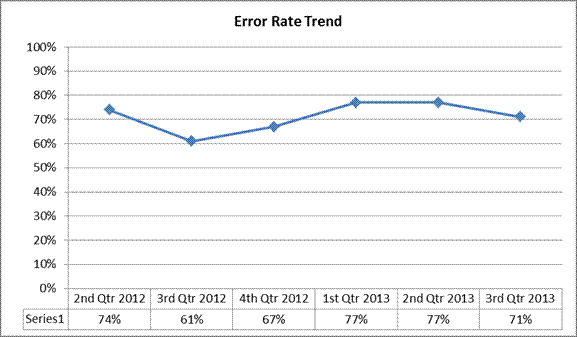
Non-response Rate to Additional Documentation Requests – 26%
An analysis of the claim denials from the DCR review shows that the top 10 reasons a determination was made not to pay the claim were:
- The documentation received did not include a copy of a valid refill request.
- The documentation lacked a current (within six months prior to the date of service being reviewed) test log or physician record (narrative statement in the progress notes, etc.) documenting the frequency at which the beneficiary was actually testing.
- Proof of delivery was not provided or was incomplete.
- The date of service entered on the claim did not match the shipping date (items delivered via mail/shipping service).
- The date of service under review was prior to the signature date on the detailed written order and the documentation received did not include written documentation of a preliminary dispensing order.
- A copy of the detailed written order was not received.
- The date of service on the claim does not match the date the beneficiary or a representative signed for delivery (items personally delivered to beneficiary).
- The ordering practitioner did not personally date his/her signature on the detailed written order.
- The detailed written order was missing one or more of the following elements: 1) Beneficiary's name, 2) Specific list of all items to be dispensed, 3) Specific frequency of testing, 4) Start date (if different from signature date).
- The physician's signature on the detailed written order was missing, therefore the identify and credentials of the person writing the order could not be authenticated.

